Metals Tend to Form Cations or Anions
There are ten answers to this question. Metal elements form positively charged ions called cations because they are located on the left side of the periodic table.

Solved Question 6 1 Point Why Do Metals Tend To Form Chegg Com
They are REDUCING and tend to do.

. Halogens always form anions alkali metals and alkaline earth metals always form cations. Atoms of the metallic elements have relatively weak attractions for their electrons so they tend to lose electrons and form monatomic cations cations composed of one atom such as Na. Every element in the first column forms a cation with charge 1.
41K views View upvotes Related Answer Sonal Jain. The electron affinity of metals is lower than that of nonmetals. Fri Sep 24 2021 1237 pm.
Lets learn why do most metals tend to form cations. They absorb energy endothermic to lose electrons. Anions tend to be ____ and cations tend to be ____.
Kainath Kamil Dis 2K Posts. Sodium1 is a monoatomic monocation obtained from sodium. Groups 1 and 2 are called the alkali metals and alkaline Earth metals respectively.
It is an alkali metal cation an elemental sodium a monovalent inorganic cation and. Second elements that live in the first two columns and the last three columns of the period table show a defininte trend in charges. The non-metals are to the upper right of the Periodic Table as WE face the Table.
Since metals lose electrons they form cations. Yes the cation is the metal because metal tend to lose electrons and the anion is the non-metal. By writing the name of the cation followed by the name of the anion.
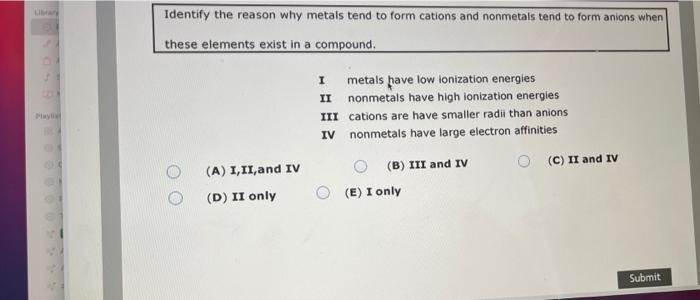
While the chlorine anion Cl- is formed by the gain of an electron by the group 7 non-metal chlorine atom. For example in NaCl the cation Na is formed by loss of an electron from the group 1 metal sodium. 1 metals have low lonization energies II nonmetals have high ionization energies III cations are have smaller radii.
Atoms with strong attractions for electrons are non-metals and tend to form anions negative ions. This is actually one of the chemical properties of metals and nonmetals. Identify the reason why metals tend to form cations and nonmetals tend to form anions when these elements exist in a compound.
The metallic elements generally form cations and the non-metal elements typically form anions. Metals like to lose valence electrons to form cations to have a fully stable octet. Now due to their configurations metals tend to lose electrons whereas non metals tend to gain them.
The most accurate or helpful solution is served by Yahoo. Answer 1 of 2. 100 or so elements.
View the full answer. Energy in a reaction is unfavorable and brings a gain in stability due to the electrostatic attractions between product anions and cations in its formation. These elements all have valence electrons in an s orbital.
Typically since metals form cations and nonmetals form anions we are able to assume that in a salt the cation will be the metal and the anion will be the nonmetal. Well have a look at the Periodic Table. Now classically metals are regarded as electron-rich particles.
It has a role as a human metabolite and a cofactor. Metals tend to form cations while nonmetals tend to form anions. Nonmetals like to gain electrons to form anions to.
Most other metals form cations eg. This gaining or losing of electrons enables bond formation and stability. What ions do metals tend to form.
Is na1 a cation or anion. Metals tend to form cations and nonmetals form anions because metals lose electrons and non metals gain electrons What types of ions do the metallic and the nonmetallic elements form. Iron silver nickel whilst most other nonmetals typically form anions eg.
Positive ions cations is they lose electrons or negative ions anions. And MOST elements are metals. Metals tend to give up electrons and form cations.
The cation is the same as the metal name the anion is the name of the nonmetal w the suffix changed to -ide Sn and Pb from the p block will _____ Form more than one type of ion and behave like transition metals. Atoms of the nonmetallic elements have relatively strong attractions for electrons so they tend to gain electrons and form monatomic anions anions composed of one. Generally metals tend to lose electrons to form cationsthat shows they have lower.
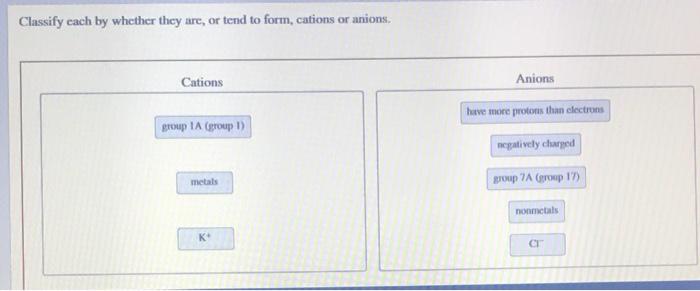
Solved Classify Cach By Whether They Are Or Tend To Form Chegg Com
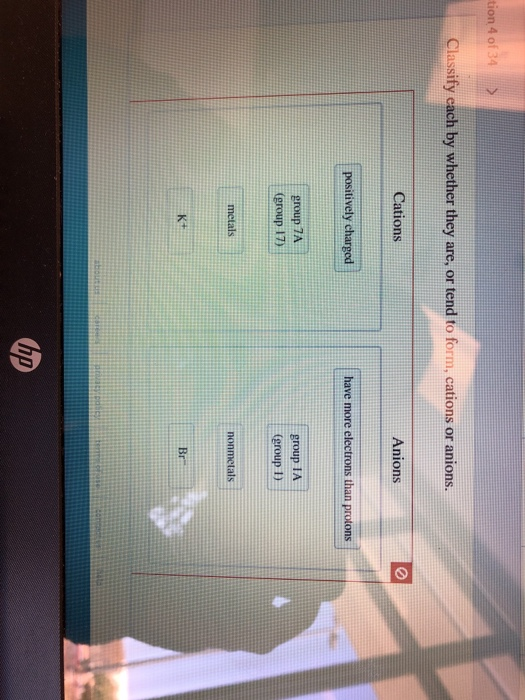
Solved Tion 4 Of 34 Classify Each By Whether They Are Or Chegg Com
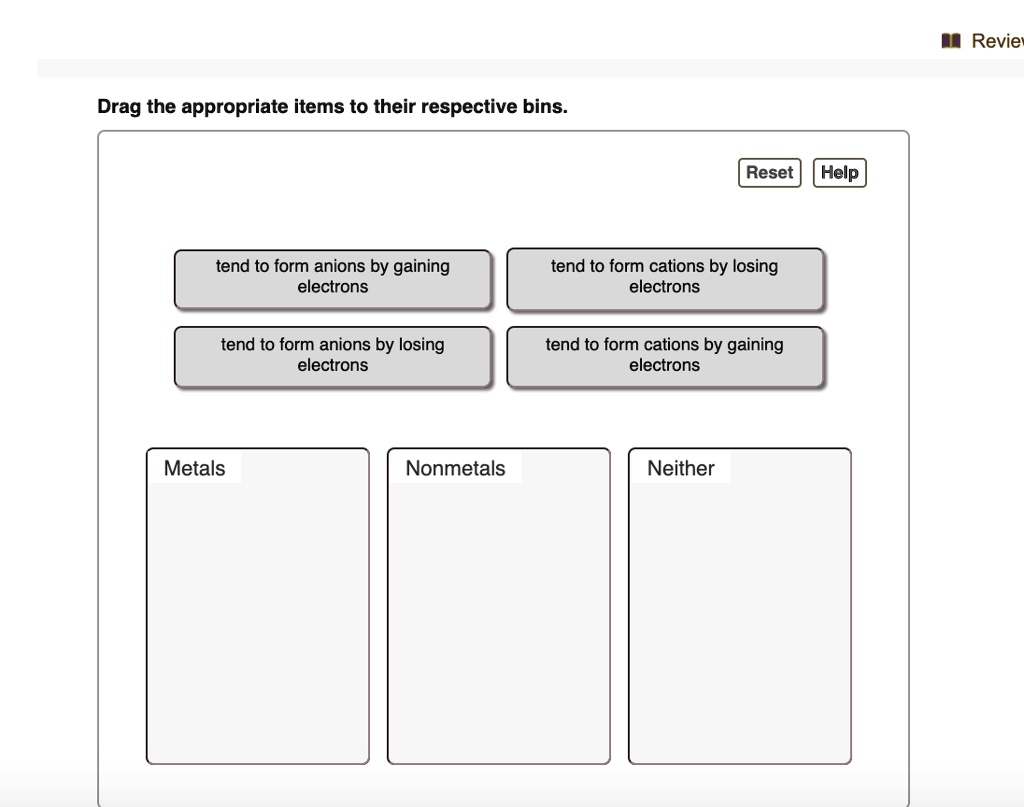
Solved Revie Drag The Appropriate Items To Their Respective Bins Reset Help Tend To Form Anions By Gaining Electrons Tend To Form Cations By Losing Electrons Tend To Form Anions By Losing Electrons
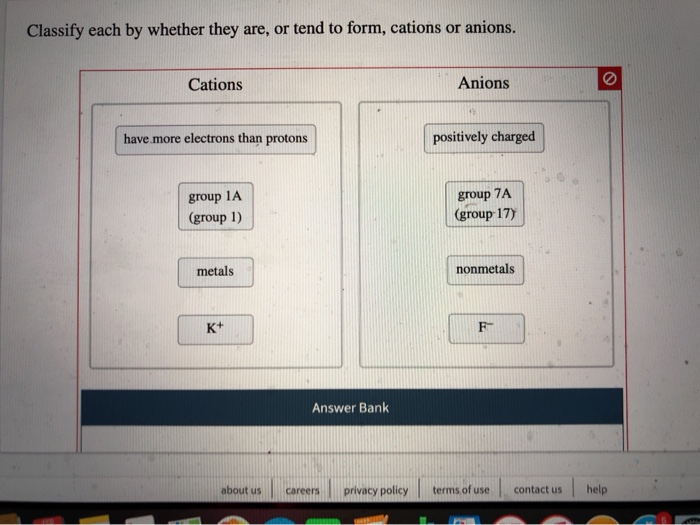
Solved Classify Each By Whether They Are Or Tend To Form Chegg Com

Properties Of Metal Nonmetals And Metalloids Metals Versus Nonmetals Differences Between Metals And Nonmetals Tend To Revolve Around These Properties Ppt Download
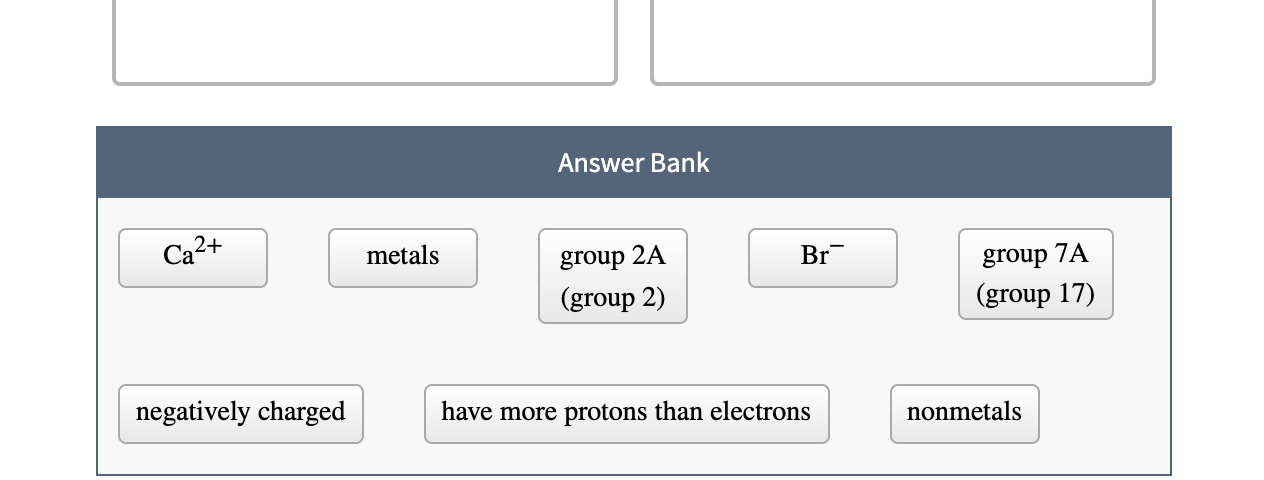
Solved Classify Each By Whether They Are Or Tend To Form Chegg Com

Which Of The Following Tends To Form Anions When Bonding With Other Elements Lisbdnet Com

Oneclass Question 2 Of 10 Multiple Choice Which Statement Is O A Halogens Tend To Form Anions Wit
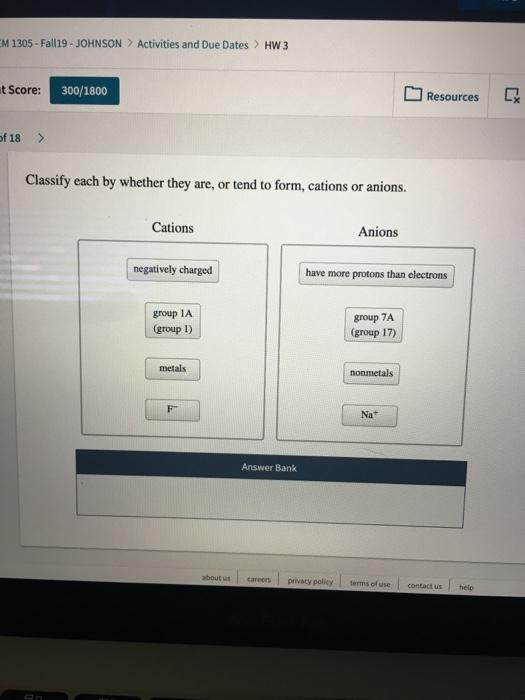
Solved 1305 Fall19 Johnson Activities And Due Dates Chegg Com
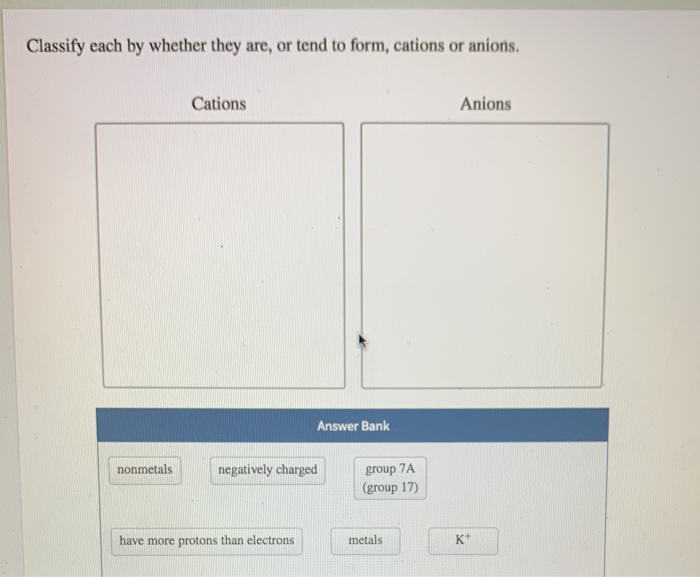
Solved Classify Each By Whether They Are Or Tend To Form Chegg Com

Solved Classify The Following By Whether They Are Or Tend Chegg Com
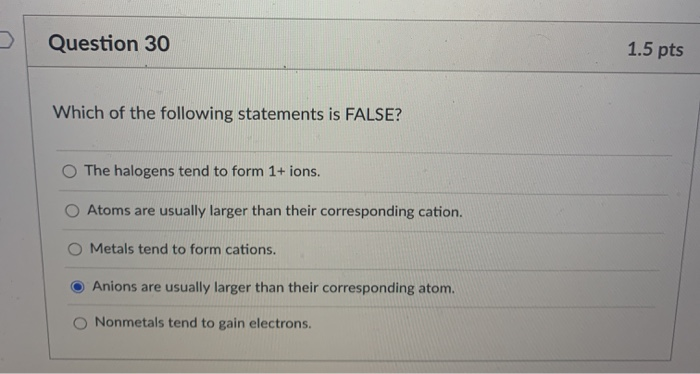
Solved Question 30 1 5 Pts Which Of The Following Statements Chegg Com
Solved Identify The Reason Why Metals Tend To Form Cations Chegg Com
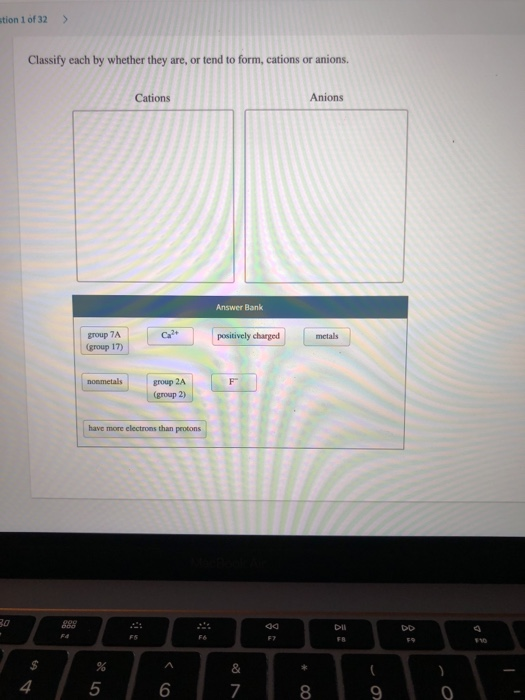
Solved Stion 1 Of 32 Classify Each By Whether They Are Or Chegg Com
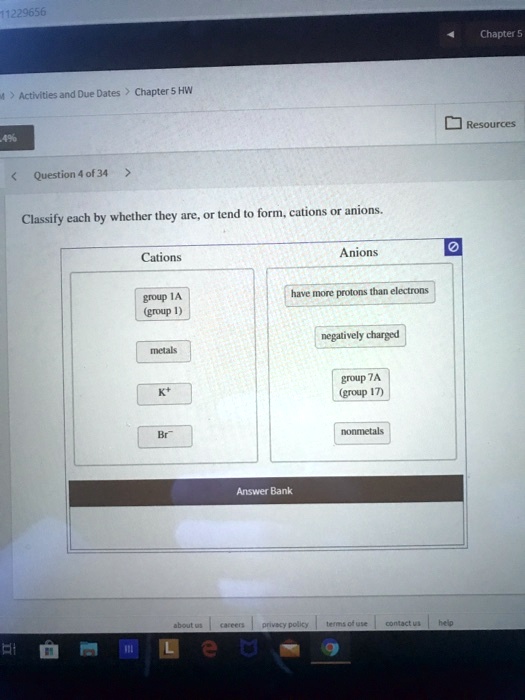
Solved 11229656 Chapter Activities And Due Dates Chapter 5 Hw Resources Question 4 0f 34 Classify Each By Whether They Are Tend Form Cations Or Anions Cations Anions Have More Protons Than Electrons
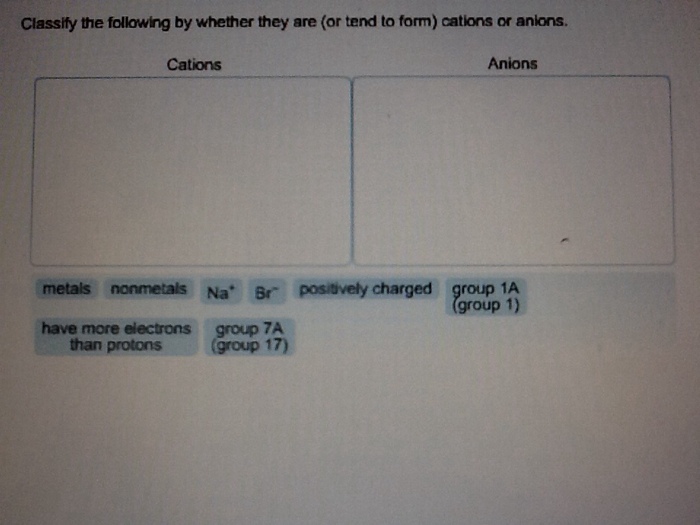
Solved Classify The Following By Whether They Are Or Tend Chegg Com
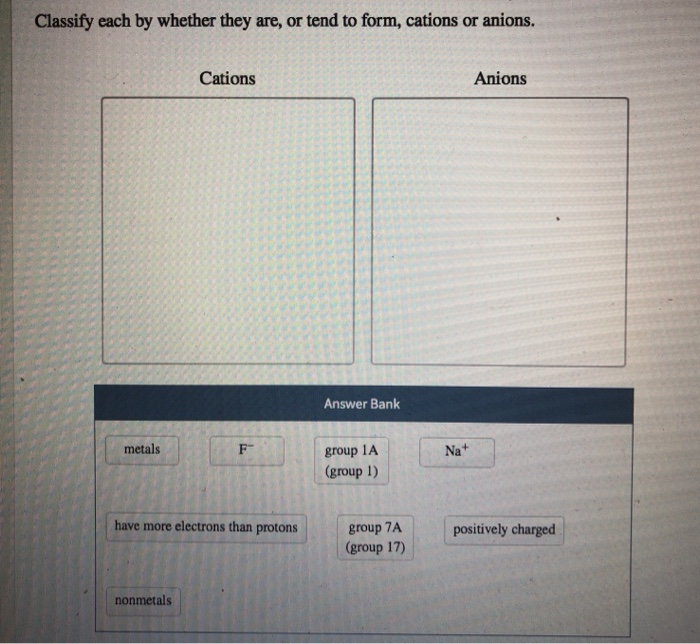
Solved Classify Each By Whether They Are Or Tend To Form Chegg Com

Solved Classify Each By Whether They Are Or Tend To Form Chegg Com
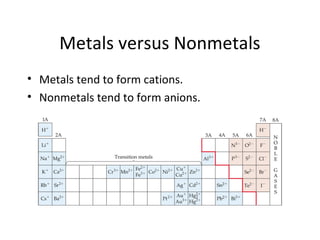
Comments
Post a Comment